RE104 Safety and Efficacy Study in Adjustment Disorder in Cancer and Other Medical Illnesses
RECRUITMENT FOR STUDY
Study Sponsored by Reunion Neuroscience
A Phase 2, randomized, double-blind, parallel-group, dose-controlled study to determine if treatment with a single dose of RE104 for Injection reduces depressive symptoms or depressive symptoms mixed with anxiety symptoms in participants with Adjustment Disorder due to cancer or other illnesses such as Amyotrophic Lateral Sclerosis (ALS), Multiple Sclerosis (MS), Parkinson’s Disease (PD) or Idiopathic Pulmonary Fibrosis (IPF) as compared to active-placebo.
Introduction and Rationale for Study
Adjustment disorder is a mental health condition characterized by a significant emotional or behavioral response to a stressful life event or change, such as the diagnosis of a medical condition or illness. Common symptoms include anxiety, depression, withdrawal, agitation, and aggression. Individuals may also experience anger, difficulty concentrating, and strained relationships. Adjustment disorder is particularly prevalent among cancer patients, and there are currently no approved treatments.
RE104 for Injection (RE104) has potential as an effective treatment for Adjustment Disorder as a novel serotonergic antidepressant therapeutic because of the potential for rapid symptom relief, as well as sustained response after a single short treatment.
Purpose and description
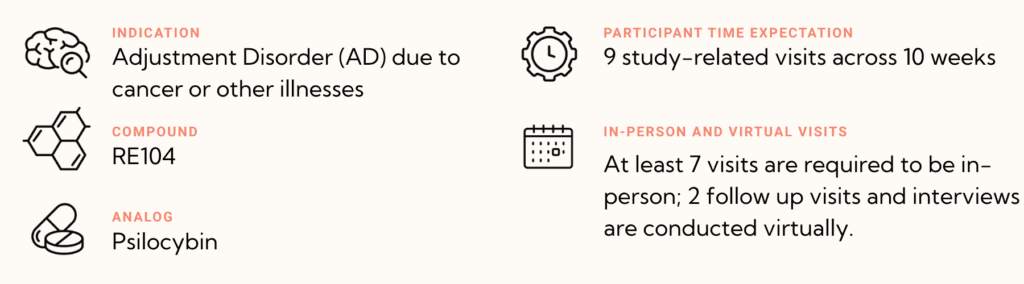
The purpose of this study is to evaluate if 30 mg RE104 reduces depressive and anxiety symptoms compared to 1.5 mg RE104 in patients with Adjustment Disorder due to cancer and other medical illnesses (Multiple Sclerosis (MS), Amyotrophic Lateral Sclerosis (ALS), Parkinson’s Disease (PD), and Idiopathic Pulmonary Fibrosis (IPF)).
Participation is estimated to last approximately 10 weeks including in-person and virtual visits:
- Screening & Preparatory Visits (2)
- Dosing Session (1)
- Follow-Up Visits (6)
- 4 in-person
- 2 virtual
- Entry Interview prior to dosing (if selected
in-person or virtual) - End of Study Interview (if selected in-person at the last follow-up visit or virtually)
Important Considerations
- The study’s procedures and therapy are at no cost to the participant. Eligible participants can receive up to $768 in compensation for study activities.
- Eligible participants will be randomized in a 1:1 ratio to receive a single subcutaneous (under the skin) injection of either 30 mg RE104 or 1.5 mg RE104. Eligible participants will be randomized in a 1:1 ratio to receive a single subcutaneous (under the skin) injection of either 30 mg RE104 or 1.5 mg RE104. Participants, research staff, and session monitors are all blinded. A limited number of personnel who prepare and administer the study drug will be unblinded.
- Key eligibility criteria include:
○ Males and females ages 18-80
○ 4 week history or greater of adjustment disorder due to cancer, MS, ALS, PD, or IPF
○ Projected life expectancy of greater than or equal to 6 months
○ No significant risk of suicide
○ No other medically significant conditions that would render the subject unsuitable to participate - Study drug will be administered in private, specifically designed rooms, with physicians and medical staff present on site and constant support from trained and experienced
mental-health practitioners.
- Participants may remain on SSRIs, SPARIs, SMSs, or SNRIs if they have been on a stable dose for at least 30 days prior to screening and do not plan to change their dosage until the end of the study. Participants MUST NOT change or discontinue ANY medication without the physician’s approval. If necessary, the study physician will consult with patients’ providers.
- Participants should not be pregnant, nursing or planning a pregnancy, and must agree to use a highly effective method of birth control during the entire course of the clinical trial.
- Risks to participation will be reviewed extensively during the screening and informed consent process.
- Risks to participation will be reviewed extensively during the screening and informed consent process.
To inquire about participation in the Rekindle Study, apply below:
For more information about the study: